An intelligence platform for unlocking insights for Clinical
Trials globally
At TrialOptima, we believe in advancing healthcare through data-driven insights. Our comprehensive database and intelligence platform is designed to revolutionize the landscape of clinical trials globally, providing researchers, pharmaceutical companies, CROs and healthcare professionals worldwide, with cutting-edge tools they need to optimize every aspect of the trial process.
TrialOptima understands that each stakeholder involved in Clinical Research has distinct requirements: From refining trial design using historical data to locating investigators with specialized expertise, identifying relevant trial sites based on disease prevalence, to accessing detailed endpoint information from past trials, TrialOptima offers trial insights and solutions to meet “Your Needs, Your Way.”
The platform empowers you to pinpoint trial information precisely according to your search criteria. Additionally, it offers insights into a broader spectrum of associated information beyond your initial focus area, empowering you to craft a more comprehensive blueprint for your trial.
|
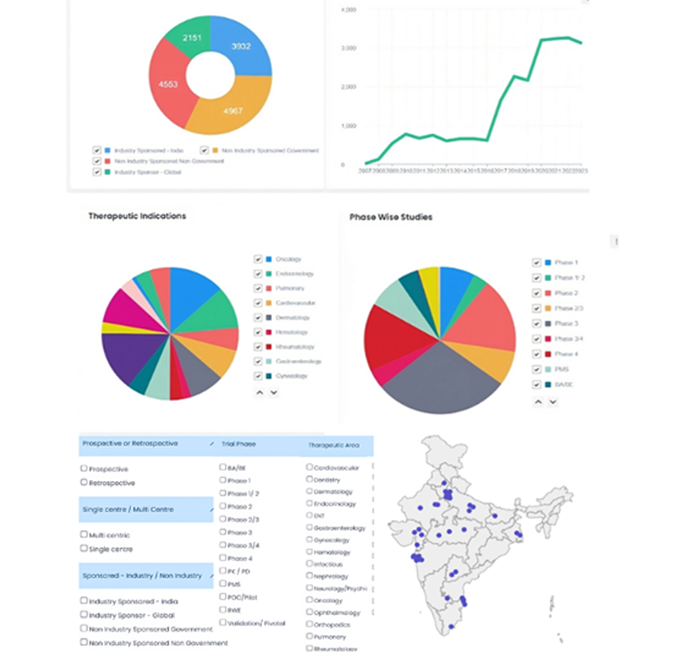
Comprehensive Global Data Coverage Access the world's most comprehensive repository of clinical trial data spanning 194 countries, featuring integrated information from several public databases, all meticulously curated and updated in real-time. |
|
|
Advanced filters and analytics Refine your search criteria with advanced filtering options, enabling precise targeting and identification of trials that meet your specific requirements, with insights drawn from global clinical research trends and patterns. |
|
|
Regulatory Pathway Intelligence Analyze regulatory pathway journeys, timelines and draw insights from our regulatory data with our integrated SEC regulatory pathway data from CDSCO for India. |
|
|
Streamlined Global workflow Simplify your trial management with our intuitive interface, designed to enhance efficiency and productivity throughout the trial lifecycle, across multiple countries and regions. |
|
|
AI-Powered Intelligence Engine Use the power of Generative AI and Large Language Models (LLMs) to derive deeper insights, summarize complex trial data, and uncover hidden patterns across global datasets—enabling faster, smarter, and more informed decision-making. |

Effortlessly find relevant trials across global databases and explore detailed information, including study design, endpoints, recruitment status, and enrolment data.

Compare trial designs identify trends, and benchmark against industry standards for actionable intelligence that drives strategic decision-making.

Evaluate site feasibility with ease through our platform, accessing comprehensive data on site capabilities, patient populations, and logistical considerations, facilitating informed decision-making during trial planning.

Access detailed profiles of key stakeholders including sites, investigators, and sponsors empowering efficient collaboration and strategic planning throughout the trial lifecycle.

Join the TrialOptima Community
Unlock the full potential of global clinical trials intelligence with our flexible pricing options designed to scale with your research needs and international ambitions.
For detailed pricing information, custom enterprise solutions, or to schedule a personalized demo of our global platform capabilities, please contact us at trialoptima@jcdc.co.in. Our team is ready to help you harness the power of worldwide clinical trials data.
Ready to elevate your clinical trials with TrialOptima?
Sign up for a demo today and experience the power of intelligent insights for yourself.
